The first rule of the DFTBquiz is that the approach to each particular case and patient is not dogma, nor is it the only way in which the case can be safely managed in our virtual ED. There are numerous ways to approach critical illness. As long as the applied clinical treatment passes both the evidenced based medicine and family litmus then we have nothing to fear apart from the disease process itself.
So how would the DFTB team at Bubbles Central Hospital approach the child with life threatening bronchospasm, altered sensorium that has a pneumothorax and an SVT?
If you missed the original question – check it out here
This has been the second most successful #pedsicu f#ridayquiz to date with >30k impressions and answers from 29 different countries! It was a complex case of common pathologies amalgamated in one patient – status asthmaticus with a pneumothorax and SVT.
We outline the DFTB team’s take on the case and how we would approach it if we had this patient in our own resus bay. Please note this is not the only way to approach the patient but rather what our consensus is as to how to prioritize clinical issues and minimize risk in this patient by using a rational, evidence-based and pharmacologically prudent approach. There were numerous excellent answers from across the globe. Here are a few highlights…
Things to consider are:
- What is immediately the most life-threatening pathology? The pneumothorax? The SVT? The severe bronchospasm?
- Why does the child have lactic acidosis?
- Is it really an SVT or is it a tachycardia, exacerbated by nebulized beta-agonists? What risks are posed by any intervention we undertake?
- How do we minimize the risks identified above?
- What drugs should we use for intubation and what how do we maintain anaesthesia thereafter?
1. What is immediately the most life-threatening pathology?
It is clear that this child is at high risk of cardiorespiratory arrest if we do nothing.
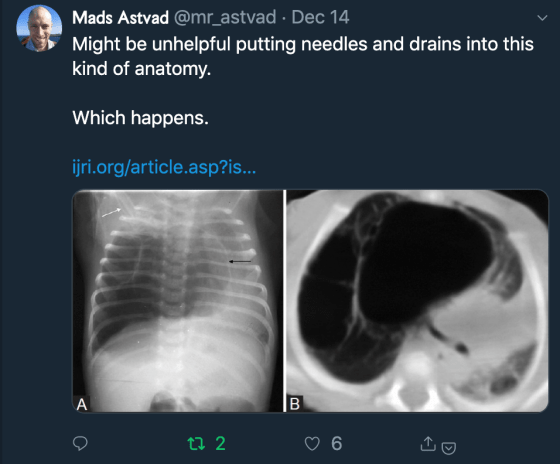
Clues to that are hypoxia, hypercarbia (especially in the context of altered sensorium)[1]; air trapping to the extent where a pneumothorax has developed (a known complication of asthma)[2] and the lactic acidosis, which in this case is likely to be secondary to a combination SVT leading to myocardial hypoperfusion and the respiratory muscles tiring (more on that later).
On the ABCDEFG approach (Airway, Breathing, Circulation, Disability, Exposure, Fluids, Glucose) we are taught to approach airway first. This failsafe approach may work well in most clinical emergencies but in this case, intubating before achieving cardiorespiratory stability is likely to put the patient in an even stickier situation. Breathing (i.e. adequate oxygenation) is likely to be the first pathology to lead to cardiorespiratory arrest. That needs to be addressed first. The SVT is likely to cause considerable instability during intubation; this is superimposed to the pre-existing high risk of adverse events that accompany life-threatening asthma[3]. So the SVT needs to be cardioverted prior to intubation if possible.
Furthermore, the risk of converting a pneumothorax to a full-blown tension pneumothorax by attempting to intubate first is significant. Most modified RSI methods include a bag and mask ventilation technique. The application of positive pressure ventilation either before or after the ETT is in place –once the patient is established on a ventilator- risks changing the nature of the pneumothorax from a simple one to a life-threatening tension-type one[4].
In this case, therefore, airway stabilization – although high on the list of priorities – should come after we have optimized breathing and circulation (unless the patient arrests beforehand).
2. Why does the child have lactic acidosis?
The latter is important to understand and differentiate in someone who has been receiving a beta-agonist.
In the context of asthma lactic acidosis may be due to overproduction and/or inadequate clearance of lactic acid. Therefore, lactic acidosis in a child with severe bronchospasm could result:-
-
-
- if patients were in occult shock
- if produced by tiring respiratory muscles (i.e., respiratory muscle oxygen demand outstripping oxygen supply)
- if produced by the lung parenchyma
- if changes in glycolysis were caused by beta-agonist administration.
- lactic acid could also be under metabolized by the liver
-
In our case the patient did not receive any IV salbutamol and only a couple of nebulizers; pharmacogenic lactic acidosis is therefore unlikely.
Much more likely is a lactic acidosis as a result of tiring respiratory and cardiac muscles. The latter is especially important to recognize in the context of an SVT. The myocardium perfuses during diastole[6]. If the HR is 300, the diastolic time is minimal, so there isn’t much time for the myocardium to be adequately perfused.
Tired respiratory and cardiac muscles make for a very high-risk intubation process.
3. Is it really an SVT or is it a tachycardia, exacerbated by nebulized beta-agonists?
It is tempting to think that such a significant tachycardia has been caused by a combination of factors: the patient is hypovolaemic, the patient is stressed, we gave him a couple of salbutamol nebs – and so on.
How can we differentiate a sinus tachycardia from an SVT?
Most textbooks will empirically state if the HR is >210-220 then the rhythm’s is more likely to be SVT, if it is <200-210 then it is likely to be sinus tachycardia.
This is loosely true but not always, especially in the context of paediatrics where we have different HR norms for each age.
Beat-to-beat variability is important in differentiating SVT from sinus tachycardia. Whilst in SVT each (P) QRST complex looks the same as the one after it, in sinus tachycardia each PQRST complex is different. A 12 lead ECG will help you ascertain this more accurately.
The presence of P waves is another determining factor. A true SVT oughtn’t to have P waves preceding the QRS complex, whereas in a sinus tachycardia a P wave is usually present. This is often tricky to differentiate in practice, especially if the ECG or cardiac monitors are tuned onto real-time speed. The best trick to apply is to slow the monitors down enough. This will slow down the speed of the PQRST complexes, allowing us to better visualize the P wave.
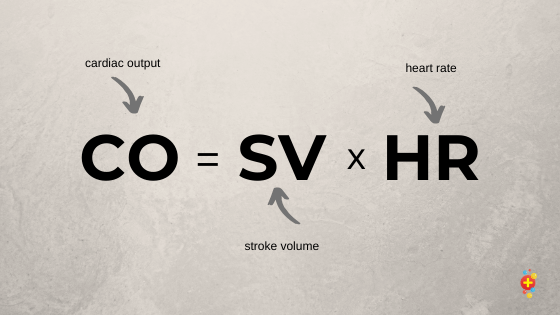
Vagal manoeuvers and pharmacological therapy if there is uncertainty about the cardiac rhythm is poor practice and should be avoided. Cardiac output equals stroke volume times heart rate (CO= SVxHR). If we try to slow down the heart in the context of very fast sinus tachycardia with drugs or by stimulating the vagus nerve we will drop the cardiac output and put the patient at risk of a cardiac arrest. We always need to be sure of the rhythm before any intervention.
If you are still uncertain, a reasonably safe bedside test would be to give 10ml/kg fluid bolus (ideally balanced solution) and keep an eye on the monitor whilst it’s infusing. If it is an SVT the HR will not budge. If it is sinus tachycardia, you are much more likely to see some slowing down of the rate.
4. What risks are posed by any intervention we undertake?
The risks of intubating someone with pneumothorax have been outlined above.
PPV can change a stable, small pneumothorax into a life-threatening tension pneumothorax. This dictates that we should ideally put a temporary chest drain in to decompress the thorax prior to intubation.
The other risk in optimizing breathing in this scenario is an exacerbation of the SVT by giving IV bronchodilating agents that are known to have a potent chronotropic effect. Both aminophylline [7] and salbutamol [8] are known to be chronotropic, but evidence would suggest that aminophylline causes less of a chronotropic effect than salbutamol[9]. With that in mind, loading with IV aminophylline in order to break the bronchospasm spiral would be the best (or least bad) option.
Also worth noting that MgSO4 is a potent vasodilator, so if we intend to use it in this setting to optimize bronchodilation it needs to be done as a low infusion (over 25-30 minutes)
The risks we may encounter whilst in improving circulation prior to intubation are twofold.
Firstly, in addressing cardioversion, adenosine is the most commonly used agent in treating SVT pharmacologically. A known side effect of adenosine, however, is bronchospasm[10]. There is little high-quality evidence to assess the effects of adenosine on asthmatic airways. What little evidence there is (and the evidence is nearly all from adult subjects) would suggest that adenosine is safe to use in patients with reactive airways[11],[12].
Secondly, this patient is likely to have a degree of dehydration. This degree of tachypnoea and work of breathing increases fluid loss through the respiratory tract. The degree of tachycardia also suggests a hyper-metabolic demand, again suggesting increased fluid consumption. It would, therefore, be prudent to give this patient some volume prior to intubation. As the patient already has metabolic acidosis, (ab)normal saline would be a poor choice. The chloride content is likely to increase chloride levels leading to a worsening metabolic acidosis [13], which in turn would worsen myocardial contractility [14],[15]. Balanced solutions (Plasmalyte 148 or Hartmann’s) are by far more physiologically appropriate and unlikely to exacerbate the metabolic acidosis [16],[17] and therefore preferred in this instance.
5. How do we minimize the risks identified above?
We have alluded to a lot of the steps in the analysis above. The main objective is to optimize oxygenation and primum non-nocere.
Bronchodilation prior to intubation is key. In this case, it is reasonable to go “all-out” and load with IV aminophylline, IV Hydrocortisone, IV Magnesium and a triple agent nebulizer (repeat if needed) consisting of salbutamol, ipratropium, and adrenaline (croup dose) to try and minimize air trapping by opening up the airways.
A temporary chest drain is important. This will help with pre-intubation oxygenation and reduce the risk of a peri-intubation tension pneumothorax from developing.
Cardiovascular stabilization is also important prior to intubation. Volume resuscitation prior to intubation is best done with a balanced solution (as outlined above) and –if anaemic- possibly blood as that would help with the overall oxygen-carrying capacity and give the patient more reserve. It is important to remember that this should be done in 10ml/kg aliquots because a high proportion of children with SVT will have concomitant congenital anatomical abnormalities. Give the fluid, assess response, check for rhonchi and hepatomegaly, and repeat as necessary. It is possible that the patient may still need cardiovascular support after intubation.
Which inotrope is best will be dictated by whether or not we have managed to successfully cardiovert (by vagal maneuvers first, by incremental doses of adenosine second and by DC cardioversion third). The inotropes need to be pre-drawn, prior to intubation so that we can start them quickly. This is not a scenario where we should be playing catch-up and preparation is key.
IV adrenaline would be a strong favorite in the usual asthmatic, not least because it has potent bronchodilatory effects and is reasonably safe to use in asthmatics[18]. If we have managed to stop the SVT then there would be a strong argument to favour this. Adrenaline, of course, is also a potent chronotrope, so we should; on balance avoid it in someone with SVT. Noradrenaline is the least chronotropic out of our inotrope choices, so if we are still in SVT or we think that the patient is at high risk of reverting back into SVT then it would, on balance, be our best choice. Have a low threshold for inserting an IO if you don’t have enough large-bore access.
6. What drugs should we use for intubation and what how do we maintain anaesthesia thereafter?
There is a long-standing truism in the art of rapid sequence intubation that says, “there is no such thing as a cardiostable induction”. This is especially true in the intubation process of critically ill children. All induction agents tend to vasodilate and cause a blood pressure drop. Couple that with the vagal stimulation caused by the laryngoscope and you can see why RSI is tricky business.
Arguably the least cardio-unstable combination of drugs in this setting would be ketamine (1-2mg/kg),fentanyl (1mcg/kg), and rocuronium (1-2mg/kg). Ketamine has the added benefit of being a bronchodilator so it would definitely help in reducing the bronchospasm[19].
Intubating using sevoflurane may also be attractive for experienced anesthetists, not least because of the potent bronchodilatory effect that it can offer us[20]. This would still be my second choice however, because of how much vasodilation and blood pressure drop it may cause.
Always be prepared for adverse events during intubation. In this case, our chest drain needs to be in first, we need some inotropes pre-drawn as well as some volume in case the BP drops. A favorite trick of mine is using dilute adrenaline as a bolus to improve BP or HR or both should they drop during intubation.
The dilution is essentially tenfold of the resuscitation dose. Take the resus dose, dilute it with 10 ml of saline and you can bolus the eventual solution in 1ml aliquots. This is a superior drug when compared to commonly used atropine as it addresses also the BP drop and not just the HR drop.
Maintenance of anaesthesia is often with continuous infusion of morphine and midazolam. In this case, those agents would not be the best choice. Morphine is known to increase histamine release and is therefore likely to exacerbate bronchospasm and peripheral vasodilatation. Fentanyl, as a continuous infusion, is proven to cause less histamine release and is, therefore, a superior choice in this case[21].
Coupling the fentanyl with a ketamine infusion (instead of midazolam) would also be preferable, mainly because of ketamine’s bronchodilatory effects. For doses /rates and dilutions of these pharmacological agents fill in and print the drug chart on crashcall.net or the one provided by your regional paediatric critical care transport team.
So what plan would go up on the PED resus board?
- Optimize B and C first. Prepare Airway trolley (including 4, 4.5 and 5 cuffed ETT) and draw up 10ml aliquots of Plasmalyte dilute adrenaline. Draw up noradrenaline and adrenaline for infusions if needed.
- Break the bronchospasm cycle. IV aminophylline, slow IV MgSO4, triple neb (adrenaline, salbutamol, ipratropium). Temporary chest drain –and prepare for a more robust one after intubation.
- Confirm rhythm. 10ml/kg fluid volume, vagal maneuvers, incrementally increasing doses of adenosine until Cardioversion 100mcg/kgè200mcg/kgè 300mcg/kgè500mcg/kg. If adenosine fails for DC Cardioversion. Ideally prior to intubation.
- 1-2mg/kg ketamine, 1mcg/kg fentanyl, 1-2mg/kg Rocuronium; maintain anaesthesia with ketamine and fentanyl infusions (crashcall.net doses/rates)
- Empirical cover, include cover for atypical infections: Ceftriaxone + Clarithromycin. If flu possible consider Oseltamivir.
- Avoid 0.9%Saline, 10ml/kg aliquot of Plasmalyte or Hartman’s, if anaemic consider blood. Reassess after every bolus (liver size and rales).
- Keep an eye, likely to rise (stress response, steroids, salbutamol) unlikely to need treatment even if high.
Remember, this is just the DFTB team’s approach. There are numerous ways to skin a cat; if you have an alternative way we are keen to hear it!
References
[1] Holley, Anthony D., and Robert J. Boots. “management of acute severe and near‐fatal asthma.” Emergency Medicine Australasia 21.4 (2009): 259-268.
[2] Porpodis, Konstantinos, et al. “Pneumothorax and asthma.” Journal of thoracic disease 6.Suppl 1 (2014): S152.
[3] Zimmerman, JANICE L., et al. “Endotracheal intubation and mechanical ventilation in severe asthma.” Critical care medicine 21.11 (1993): 1727-1730.
[4] Bacon, A. K., et al. “Crisis management during anaesthesia: pneumothorax.” BMJ Quality & Safety 14.3 (2005): e18-e18.
[5] Forsythe, Sean M., and Gregory A. Schmidt. “Sodium bicarbonate for the treatment of lactic acidosis.” Chest 117.1 (2000): 260-267.
[6] Heusch, G. “Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents.” British journal of pharmacology 153.8 (2008): 1589-1601.
[7] Urthaler, Ferdinand, and Thomas N. James. “Both direct and neurally mediated components of the chronotropic actions of aminophylline.” Chest 70.1 (1976): 24-32.
[8] Crane, J. et al “Cardiovascular and hypokalaemic effects of inhaled salbutamol, fenoterol, and isoprenaline.” Thorax 44.2 (1989): 136-140.
[9] Morice, A. H., et al. “A comparison of the ventilatory, cardiovascular and metabolic effects of salbutamol, aminophylline and vasoactive intestinal peptide in normal subjects.” British journal of clinical pharmacology 22.2 (1986): 149-153.
[10] Bennett-Guerrero, Elliott, and Christopher C. Young. “Bronchospasm after intravenous adenosine administration.” Anesthesia & Analgesia 79.2 (1994): 386-388.
[11] Burki, Nausherwan K., Mahmud Alam, and Lu-Yuan Lee. “The pulmonary effects of intravenous adenosine in asthmatic subjects.” Respiratory research 7.1 (2006): 139.
[12] Terry, Polly, and Gail Lumsden. “Using intravenous adenosine in asthmatics.” Emergency Medicine Journal 18.1 (2001): 61-61.
[13] Kellum, John A. “Saline-induced hyperchloremic metabolic acidosis.” Critical care medicine 30.1 (2002): 259-261.
[14] Cingolani, Horacio E., et al. “Depression of human myocardial contractility with “respiratory” and “metabolic” acidosis.” Surgery 77.3 (1975): 427-432.
[15] Williamson, John R., et al. “Effects of acidosis on myocardial contractility and metabolism.” Acta medica scandinavica199.S587 (1976): 95-112.
[16] Bellomo, Rinaldo, et al. “Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults.” Jama 308.15 (2012): 1566-1572.
[17] Chowdhury, Abeed H., et al. “A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers.” Annals of surgery 256.1 (2012): 18-24.
[18] Putland, Mark, Debra Kerr, and Anne-Maree Kelly. “Adverse events associated with the use of intravenous epinephrine in emergency department patients presenting with severe asthma.” Annals of emergency medicine 47.6 (2006): 559-563.
[19] Allen, Joseph Y., and Charles G. Macias. “The efficacy of ketamine in pediatric emergency department patients who present with acute severe asthma.” Annals of emergency medicine 46.1 (2005): 43-50.
[20] Schutte, D., et al. “Sevoflurane therapy for life-threatening asthma in children.” British journal of anaesthesia 111.6 (2013): 967-970.
[21] Rosow, Carl E., et al. “Histamine release during morphine and fentanyl anesthesia.” Anesthesiology 56.2 (1982): 93-96.







Is it possible to see the original scenario? (the link doesn’t work)
Thank you!