As I transition into my new(-ish) role as an emergency physician in a department that sees only the occasional child I worry that my skills are becoming degraded. I am sure that I am not the only one.
So how many critical procedures do clinicians actually practice? Take a look at this paper from the group at Monash:
This retrospective chart review looked at every paediatric attendance that required a resuscitation cubicle in three Victoria hospitals in 2013. There were a total of 54,633 presentations of which 5,895 (10.7%) were assessed in a resus cubicle. Only 37 children required any sort of critical procedure. This equates to around 7 per 10,000 presentations. The researchers then looked at what sort of procedures were performed. Take a look at the table below to see what we’re talking about.

Only 53 of these critical procedures were performed over the course of the year and these were performed by only a small group of emergency physicians. 83% of doctors working at these campuses did not perform a single critical paediatric procedure. I work part time in hospital (0.5 EFT) and may see no more than 20 children a week. Going by these figures it might take me 17 months to perform a single critical procedure. This concerns me.
(Ed. note: Luckily for those living in my part of Melbourne I’ve reached my quota already so everyone can breathe easily.)
Even then we might not be any good at them anyway.
Endotracheal intubation
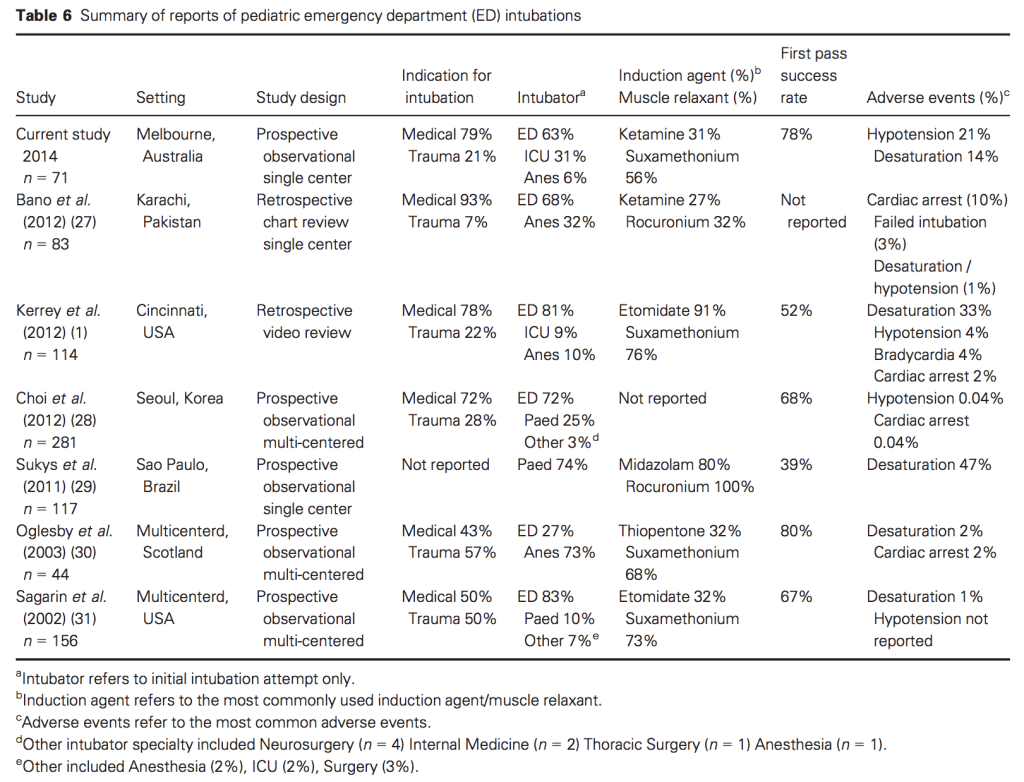
Endotracheal intubation of children is a rare event. If we look at Melbourne’s Royal Children’s Hospital, they performed 71 intubations in 66 patients over the same year time period as the Monash study above. We covered this study in our very first paper for Emergency Medicine Australasia. Their first pass success rate was 78% with a number of adverse events recorded. When hypotension or desaturation were taken into account, only 49% of attempts at intubation were without problems. This did not seem to be related to the level of experience of the intubator. Like those intubations performed at Monash, a high proportion were due to medical conditions such as status epilepticus or respiratory compromise.
Table 6 from the study suggests that we could be much better.
This post from Ross Hofmeyr suggests that we need to be performing around 75 intubations a year to maintain currency, at least in an adult population. It would take me 106 years to get that many if I just waited for nature to takes its course!
Given the high risk of adverse events cited above it would seem that I have at least a 50% chance of causing hypotension or desaturation in the only paediatric intubation I will be carrying out in the next year and a half.
What about some ‘just in time’ training? This study of 401 intubations in a PICU setting compared non-trained residents (i.e. only undertaken the mandatory training) and those that had taken a thirty minute simulation based refresher at the start of their on-call period. This consisted of 10 minutes of hands-on practice followed by twenty minutes of debriefing. Whilst there was a small procedural component, a lot of the debrief focused on communication and team management. Unfortunately the training seemed to have no impact on degree of first pass success (45.7% vs 54.7%) nor on tracheal intubation associated events (22% vs 19.9%). The only thing it seemed to have an effect on was resident willingness to participate in intubation.
So we might perform better with the aid of simulation but the question then becomes “Does this translate into clinical practice?” Walter Eppich and the team at Harvard provide some evidence in this paper from 2006 but the studies cited all have very small numbers of participants. They also include a study that focuses purely on simulation based training utilising an adult mannequin. Whilst there are some transferable elements (children are anatomically easier to intubate) the study doesn’t take into account the physiological differences between adults and children that lead to adverse events.
Task trainers may improve comfort using unfamiliar equipment but there is little evidence that they increase success rates in real life. Given the low frequency, highly challenging nature of these procedures it is unlikely that any large study is going to happy any time soon.
Intraosseous access
Intraosseous access was only obtained on 7 occasions during the study period. Studies into its success bear this out. Studies into success rates in a paediatric setting have very small numbers. This Canadian paper suggested that clinicians averaged 1.6 attempts per child with it being successful only 86% of the time.
Thoracostomy
As you might expect there is no data regarding success rates of chest tube insertion in children.
Arterial lines
Putting an arterial line in a small child can be technically challenging procedure. Only three were inserted in the Monash study and no mention is made of the technique used. An arterial line can be used to guide management but it is not the sine qua non of emergency department resuscitation. Many more are performed in the PICU than the ED. Small studies have found a success rate of around 80% using the traditional palpation technique.
CVC placement
Only one central line was inserted over the entire year studied. With such a low denominator we need to look at PICU data again to determine success rates. Using landmark technique, success has been reported at around 80%, after 2.55 attempts per child. Accidental arterial puncture took place in roughly one in four children.
So what can we do to make us more comfortable performing these low frequency procedures in a safe and effective fashion? If you base your thinking on what Twitter is saying on any given day then you would think that simulation is the answer. But is there any evidence that it actually makes a difference?
References
Nguyen LD, Craig S. Paediatric critical procedures in the emergency department: Incidence, trends and the physician experience. Emergency Medicine Australasia. 2016 Feb 1;28(1):78-83.
Long E, Sabato S, Babl FE. Endotracheal intubation in the pediatric emergency department. Pediatric Anesthesia. 2014 Dec 1;24(12):1204-11.
Horton MA, Beamer C. Powered intraosseous insertion provides safe and effective vascular access for pediatric emergency patients. Pediatric emergency care. 2008 Jun 1;24(6):347-50.
Nijssen-Jordan C. Emergency department utilization and success rates for intraosseous infusion in pediatric resuscitations. CJEM. 2000;2(01):10-4.
Schwemmer U, Arzet HA, Trautner H, Rauch S, Roewer N, Greim CA. Ultrasound-guided arterial cannulation in infants improves success rate. European journal of anaesthesiology. 2006 Jun 1;23(06):476-80.
Todres ID, Rogers MC, Shannon DC, Moylan FM, Ryan JF. Percutaneous catheterization of the radial artery in the critically ill neonate. The Journal of pediatrics. 1975 Aug 1;87(2):273-5
Chuan WX, Wei W, Yu L. A randomized‐controlled study of ultrasound prelocation vs anatomical landmark‐guided cannulation of the internal jugular vein in infants and children. Pediatric Anesthesia. 2005 Sep 1;15(9):733-8.
Nishisaki A, Donoghue AJ, Colborn S, Watson C, Meyer A, Brown CA, Helfaer MA, Walls RM, Nadkarni VM. Effect of just-in-time simulation training on tracheal intubation procedure safety in the pediatric intensive care unit. The Journal of the American Society of Anesthesiologists. 2010 Jul 1;113(1):214-23.
Eppich WJ, Adler MD, McGaghie WC. Emergency and critical care pediatrics: use of medical simulation for training in acute pediatric emergencies. Current opinion in pediatrics. 2006 Jun 1;18(3):266-71.
Mayo PH, Hackney JE, Mueck JT, Ribaudo V, Schneider RF. Achieving house staff competence in emergency airway management: results of a teaching program using a computerized patient simulator. Critical care medicine. 2004 Dec 1;32(12):2422-7.
Finan E, Bismilla Z, Campbell C, Leblanc V, Jefferies A, Whyte HE. Improved procedural performance following a simulation training session may not be transferable to the clinical environment. Journal of Perinatology. 2012 Jul 1;32(7):539-44.
Leyvi G, Taylor DG, Reith E, Wasnick JD. Utility of ultrasound‐guided central venous cannulation in pediatric surgical patients: a clinical series. Pediatric Anesthesia. 2005 Nov 1;15(11):953-8.
Alderson PJ, Burrows FA, Stemp LI, Holtby HM. Use of ultrasound to evaluate internal jugular vein anatomy and to facilitate central venous cannulation in paediatric patients. British journal of anaesthesia. 1993 Feb 1;70(2):145-8.
Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. CHEST Journal. 2011 Mar 1;139(3):524-9.
Bruzoni M, Slater BJ, Wall J, St Peter SD, Dutta S. A prospective randomized trial of ultrasound-vs landmark-guided central venous access in the pediatric population. Journal of the American College of Surgeons. 2013 May 31;216(5):939-43.



Thanks Andy
It is the bane of the existence of the “generalist”. Never enough opportunities to be excellent- yet we want the best care for our patients- a tough paradox.
Competition between us and specialists for these opportunities is an emerging feature of my world.
Great post
Challenging dynamics
Great post ( again) Andrew
I think the ‘learning curve literature’ for procedural skills would suggest that volume of simulated practice does help competence – to a point-, and more recently Chris Hicks and others have suggested that maybe a big part of that is the mental rehearsal. Procedures are motor + cognitive + affective + team
I’m afraid i am a believer that we are not staying ‘competent’ in these high acuity skills in any Australian ED settings – the ratio of procedures to practitioners (including pre-hospital) is now really low everywhere. Most FACEMs 1 intubation per year or less, unless doing retrieval/ other critical care. Our specialty probably drifting increasingly cognitive/ diagnostic rather than procedural
(Comments much more pertaining to adults as my background much less in paeds)
I suppose the question is then – why not more disasters?
Are the intubations generally straightforward ? Have CMACs/ checklist/ ApOx etc made it easier?
Chest drain/ ICC complications maybe more sensitive to this deficit. Suggest not many institutions could audit their ICC complications with any pride – again most done by practitioners with lower or less recent experience
I think some of the answer (and perhaps at least part of the way forward) is to stop taking an individual view of competence, but rather a collective / team / institutional approach ( ‘collective competence’ well described by Lorelei Lingard – Ted talk and papers on that if interested.)
I have only been able to rationalise this decreasing exposure/ experience by knowing i have expert proceduralists in the institution, and working with them.
It would be confronting, but our pleural procedures woudl be better if ALL done by a small team of 10 – 12 people who did them all across the hospital.
I know rural folks might need different answers, and it woudl be confronting for our current models of care in big hospitals, ( and sense of pride as critical care docs) . See also ‘EM as failed paradigm’ smacc debate Carley and Weingart. I want to believe Simon, but Scott nailed it
The alternative of mass credentialing and revalidation in all our procedures might not result in a improved clinical outcomes, and would be hugely expensive. I know Todd Fraser thinks differently and might call him out on Twitter for a comment.
In the meantime i’ll keep doing sim as some kind of help 🙂
Thanks Andrew, VB et al for your contribution to the debate.
There is not always a solution for every problem, so the alternative is to do the best we can under the circumstances we face.
For many of us, this means on occasion we will be required to perform a task that we are not adequately familiar with to feel comfortable. This obviously implies risk for the patient, and for us, as we may not be as skillful as others.
In most cases though, we simply don’t know. I’m not as skilled as my sister (an anaesthetist) in the insertion of an endotracheal tube, but does that matter? If she can get it in in 5 seconds, and it takes me 8, who cares? Or even if I get it in second pass, but the patient does not become hypoxic, does it really matter?
The reality is that we simply don’t know. Two things then follow :
1) Wherever possible we should find out. We should measure ourselves and benchmark our data to give us some idea of where we stand. How many do I do compared with the average intensivist? Am I having more complications that I would expect? Is there something that I can detect that helps me improve?
2) We should aim to perform at the best standard we can every single time we do it, whether we’re familiar or not. And here, we need help – perhaps this is regular refresher material. Perhaps it is online simulation. Perhaps it is regular, periodic peer review and assessment of our practice, whenever that occurs. Perhaps it is revalidation in an approved centre or course, such as the industry leading Emergency Medicine Procedures courses run by Andy Buck.
There are inevitably logistical barriers to such an approach, but the alternative is to continue the status quo and hope for the best.
High reliability organisations have successfully mitigated risk by minimising variation in practice, whether that be at an individual or system (team) level. Think standardised methods of communication as an example of the latter. It is high-time healthcare adopted a similar approach.
If you can be bothered, here’s a talk I did on these concepts recently : https://youtu.be/tOA4mmCK2O0
I take issue with the cliched comment about the lack of value in training for intubations paediatric or adult being somehow different, in and outside of the operating theatre. They are not. The technique and optimisation of the patient for first pass reliable intubation is identical no matter the geographical place of the patient. The only difference from elective to emergency work is the physiological stability of the patient. The intubation and the skillset around it is the same.
I see you point, Grant, though if you look at the skill-set of the doctors intubating in the RMH dataset then it is not just ED staff but intensivists and anaesthetists that are challenged. It still doesn’t explain how the first pass success rate for adults is much higher than it is for children.
Thanks for the post on this neglected subject. It’s something I thought about a great deal as I did a critical care fellowship and left many parts of EM behind for two years. However, on the subject of intubations, I would say we really don’t know the answer. All of the studies I’ve seen, including the ones you referenced, address the question of how many intubations one needs to become initially competent. This is very different from someone who becomes an expert and then wants to maintain competency, and I’ve never seen anything looking at that question. I will say that many academic EM attendings even in the adult world very rarely intubate.
Thank you for such thought provoking writing. From an education point of view, do you think this presents the opportunity to foster shared or rotational posts with a tertiary centre?
Definitely for those of us working in sub-tertiary places. But when you look at the data from the bigger two in Melbourne (Monash and RCH) they still don’t do a huge amount of intubations.
Going to theatre has been suggested as an option – it’s what the MICA paramedics do – but intubating a fasted, physiologically normal child for T&A’s is very, very different from dealing with the sick child in the ED.
I would encourage some time in NICU however to feel much more comfortable with providing CPAP and getting over the fear of intubating babies.
Whilst I would like to think simulation helps me mentally (and I often run through complex scenarios in my head) I am not sure there is much evidence that it actually improves practice. Perhaps Ben Lawton, Ian Summers or Vic Brazil can comment on that?